Biomaterials
background
Merz Biomaterials, a division of Merz North America, Inc.,
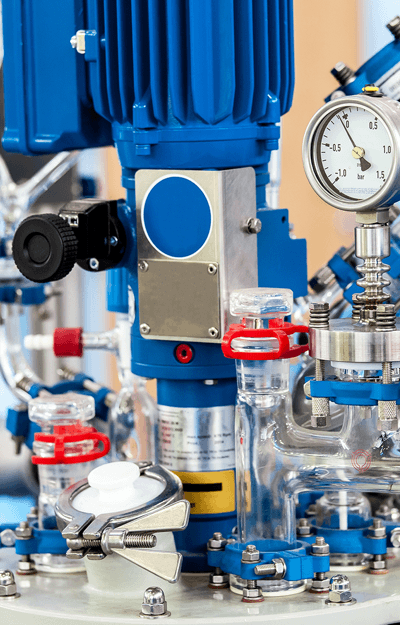
is part of the global Merz family of companies, which manufactures products sold in regulated markets around the world. Our manufactured calcium hydroxyapatite is used in globally marketed injectable implants in addition to other medical devices manufactured by our customers. Some of our customers have used our hydroxyapatite to plasma spray a coating onto orthopedic implants and as a constituent of bone screws and anchors. Merz Biomaterials is now offering our high purity* calcium hydroxyapatite to new customers for applications that need a biocompatible ceramic.
is part of the global Merz family of companies, which manufactures products sold in regulated markets around the world. Our manufactured calcium hydroxyapatite is used in globally marketed injectable implants in addition to other medical devices manufactured by our customers. Some of our customers have used our hydroxyapatite to plasma spray a coating onto orthopedic implants and as a constituent of bone screws and anchors. Merz Biomaterials is now offering our high purity* calcium hydroxyapatite to new customers for applications that need a biocompatible ceramic.

Biomaterials

Biomaterials
Why Merz Biomaterials
Our dedicated staff and manufacturing plant are able to produce and deliver large quantities of high quality, biocompatible calcium hydroxyapatite at a competitive price. Our highly educated scientists and engineers are experienced with the medical device industry and can help design and create your products using our calcium hydroxyapatite bioceramic. We are located in the Midwest of the United States with worldwide shipping capabilities. We manufacture our products in clean rooms designed to meet ISO class 8 requirements, with the capability to produce product under an ISO 13485 certified quality system and FDA cGMP.
Biomaterials